News Investigators/ Global health experts have renewed calls for Africa to strengthen vaccine research and development (R&D), regulatory frameworks, and local manufacturing capacity to combat Lassa fever and bolster preparedness.
The experts made the call in an interview with the News Agency of Nigeria (NAN) on the sidelines of a high-level health forum in Abidjan.
NAN reports that the theme of the event was “Strengthening regulatory frameworks, building production capacity, and partnerships for vaccine R&D”.
It focused on how Africa could move from dependency on foreign suppliers to building sustainable systems that ensure equitable vaccine access for its people.
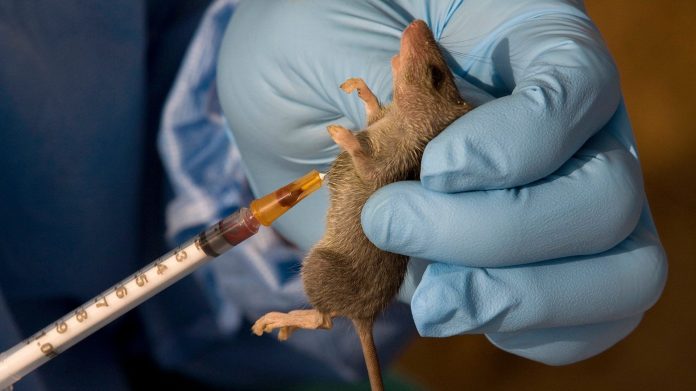
Every year, Lassa fever, viral haemorrhagic illness, threatens hundreds of thousands of lives across West Africa.
It is spread by rodents and human-to-human transmission, and continues to claim lives and overwhelm fragile health systems.
Nigeria, Liberia, Sierra Leone, and Guinea remain the most affected, but outbreaks have been reported in other countries.
Despite decades of research, there is still no licensed vaccine against the disease, leaving millions vulnerable. Current interventions focus mainly on early detection, treatment with Ribavirin, and preventive measures.
Dr Nicaise Ndembi, IVI Africa Regional Director, said that developing a vaccine was only half the battle.
“We need strong regulatory systems that can review and approve vaccines quickly, and we need local capacity to produce them on the continent,” he said.
Ndembi, stressed the importance of regional leadership in building an African vaccine ecosystem.
He cited Rwanda’s growing investments in biotechnology and vaccine manufacturing as examples of what could be achieved when West African governments prioritise long-term health security.
He said that the region could not afford to remain on the sidelines while waiting for global suppliers during outbreaks, as was seen during the COVID-19 pandemic.
Ms Oyeronke Oyebanji, Head of Lassa Engagement at the Coalition for Epidemic Preparedness Innovations (CEPI), spoke on the evolution of research on Lassa fever.
Oyebanji highlighted progress in vaccine, therapeutics and diagnostics development.
She stressed the need for sustained leadership at both national and regional levels, as well as increased resources to accelerate the journey towards a licensed vaccine.
She highlighted the importance of partnerships between governments, academia, civil society and industry in creating an enabling environment for vaccine development.
Dr Michael Lusiola of RVMC described ongoing collaborative efforts to build rapid-response vaccine manufacturing networks across Africa.
Dr Daryl Drummond of Akagera Medicines provided insights into how biotechnology companies could support Africa’s vaccine pipeline with innovation and expertise.
From a research perspective, Dr Joe Fitchett of Institut Pasteur, Dakar, stressed the role of African laboratories and institutions in conducting high-quality clinical trials.
Prof. Morenike Ukpong of Obafemi Awolowo University, Ife, reminded stakeholders that community trust was equally critical to sustaining vaccine markets.
On regulation, Dr Kwasi Nyarko of the African Vaccine Regulatory Forum (AVAREF) called for harmonised regulatory systems across African countries to accelerate approvals without compromising safety.
Meanwhile, speakers agreed that strengthening Africa’s vaccine manufacturing and regulatory autonomy went far beyond Lassa fever.
They said that it was about building resilience against the next pandemic threat.
They said that the COVID-19 pandemic exposed the vulnerabilities of Africa’s dependence on imported vaccines, where delayed access cost lives.
Experts at the session said that regional capacity would allow Africa to respond with speed, equity and justice in future health emergencies.
They said that strengthening vaccine R&D and manufacturing was not just about science; but also about sovereignty, preparedness and survival.
NAN reports that the discussions in Abidjan reflected a growing consensus that Africa must chart its own vaccine future.
By investing in regulatory frameworks, scaling up production capacity, and deepening partnerships, the region has the opportunity to curb diseases like Lassa fever and also protect itself against global health shocks.
The experts resolved that a vaccine can not remain on a shelf but must find its way to the arms of people who need it most.
NAN